Â
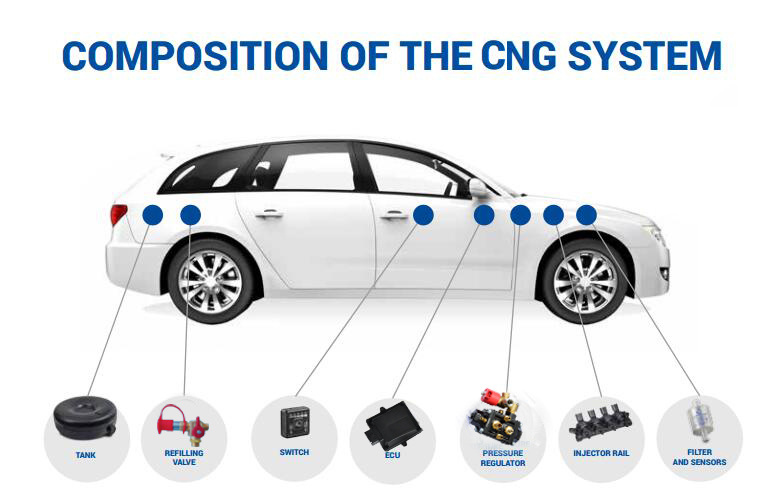
Fuel Pressure Regulator gas valve brass CTF-3 Filling valve
Brief Introduction
Â
|
SPECIFICATIONS FOR ACT CTF-1 filling valve  | |||
| Product details | Type | Stop-Check valve (Non-return valve) Â |
|
| Useable fuel  |
CNG | Â | |
| Material  |
1Cr18Ni9Ti Stainless steel | ||
| Placement  |
Between CNG tank and regulator | ||
|
Main performance parameters |
Working pressure  |
Up to 200 bar (20 MPa) | |
| Test pressure  |
250Bar(25MPa) | ||
| CNG outlet diameter to CNG tank  |
6 mm (compression fitting) | ||
| CNG outlet diameter to CNG regulator  |
6mm (compression fitting) | ||
|
Package  |
25 PCS / CTN | 10240 cm³ | |

CNG manual cylinder valve light
 Manual cylinder valve without internal venting system
Â
1. Max working pressure: 260 bar
2. Working temperature: -40°C +85°C
3. Manual security tap
4. Safeties: available without safeties or with a combination of thermal safety / excess ow / burst disk
Cylinder connections:
Â
W28.8 1"1/8UNF 1"BS341 3/4"NGT
Inlet/outlet pipe connect: G1/2
Optional Excess flow and thermal safety device;
Optional aluminum handle or plastic handle.
Â

Â
Our Company
Chengdu ACT Technology Co.,LTDÂ

Â
Located in Chengdu city, Sichuan province, China. With eighteen years development, ACT becomes one of largest CNG LPG kits manufacturers in China, mainly specializing in R&D, manufacturing CNG/LPG conversion kits for vehicles.Â
Our best seller products include CNG/LPG reducer/regulator, ECU conversion kits, injection rail, switch, emulator, pressure gauge,...Most of them are approved by ECE R110 and BV certificates.Â
Our products have been sold to more than 60 countries around the world.
Â
Â
Â
| 1.Packaging | Â A:Common Packing: 1PC +Â Plastic bag +Â Label +Â Inner Box+Export Carton 50PCS/Carton,carton size:45x35x26cm; B:Customized Packing: OEM Label, Inner Box,etc. |
| 2.Transportation | (1)By sea,by air,or by express(TNT,Fedex,UPS,DHL,Aramex,EMS etc); (2)We will do best to get good freight cost for you. |
| 3.Payment Terms | T/T, Paypal,Western Union,L/C,Escow |
| 4.Delivery Time | According to your order quantity. |
| 5.Company Type | Factory and trade integration,years of export experience; |
FAQ
Why choose us?.................................................................
1)Top Quality. Every product is made through strictly production process and quality testing, which has pass the test of BV,ISO, CE and GOST, and been accepted by clients spreading over 43 countries even including Italy and Poland.
2)1-year Warranty. All products enjoying 2Â years free of equipment maintainence warranty.
3)Free Sample. If you order a set of products for testing, we will return the freight to you from your next normal order;if you order a component for testing, we will return the sample charge to you.
4)Cost Saving. We provide long-life, shock-resistant autogas equipment with excellent quality and better performance, resulting in cost saving.
5)Quick Delivery. We promise to ship your goods with 3Â days after confirming your order, or we would like to return your payment if you asked.
Can your factory supply OEM serive?.........................................
Yes, we can. Send us the sample or the detailed speicifications of the products you want to clone →calculate the cost of opening
model →start to produce the sample for testing →continue to produce according to your feedback.
What's your MOQ, payment terms?............................................
Our MOQ :1 piece for sample order,5 pieces for normal order.
Our payment terms: T/T, D/P, L/C, Paypal, Escrow.
Â
ContactÂ

Dawson deng
Â
Chengdu ACT technology Co.,Ltd
Â
www.actong.cn   www.act-cng.com
http://actcng.en.alibaba.com/
Tel :+86-17828030170 /Â +86-15928807960
Â
Â
Tofacitinib API And Intermediates
Tofacitinib is an orally available inhibitor of Janus kinases (JAK), with immunomodulatory and anti-inflammatory activities. Upon administration, tofacitinib binds to JAK and prevents the activation of the JAK-signal transducers and activators of transcription (STAT) signaling pathway. This may decrease the production of pro-inflammatory cytokines, such as interleukin (IL)-6, -7, -15, -21, interferon-alpha and -beta, and may prevent both an inflammatory response and the inflammation-induced damage caused by certain immunological diseases. JAK kinases are intracellular enzymes involved in signaling pathways affecting hematopoiesis, immunity and inflammation.
Tofacitinib is an oral, small molecule inhibitor of Janus kinases that is used to treat moderate-to-severe rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease. Tofacitinib is associated with transient and usually mild elevations in serum aminotransferase levels during therapy, but has yet to be linked to cases of clinically apparent acute liver injury.
Tofacitinib is a pyrrolopyrimidine that is pyrrolo[2,3-d]pyrimidine substituted at position 4 by an N-methyl,N-(1-cyanoacetyl-4-methylpiperidin-3-yl)amino moiety. Used as its citrate salt to treat moderately to severely active rheumatoid arthritis. It has a role as an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor and an antirheumatic drug. It is a pyrrolopyrimidine, a N-acylpiperidine, a nitrile and a tertiary amino compound.
The chemical system of tofacitinib citrate is 3 - {(3R, 4R) - 4-methyl-3 - [methyl - (7H-pyrrolo [2,3-d] pyrimidin-4-yl) - piperidin-1-yl] - 3-oxo-propionitrile citrate. Chemical book is a Janus kinase inhibitor developed by Pfizer. On November 6, 2012, the US Food and Drug Administration (FDA) approved the substance as a drug on the market for the treatment of adult patients with moderate to severe active rheumatoid arthritis (RA) who do not respond or tolerate methotrexate.
Tofatib is suitable for adult patients with moderate to severe active rheumatoid arthritis (RA) who have insufficient or intolerable efficacy of methotrexate. It can be used in combination with methotrexate or other non biological anti rheumatic drugs (DMARD).
Tofatinib citrate is a drug for rheumatoid arthritis developed by Pfizer pharmaceutical company of the United States. It is used in adults with moderate to severe active rheumatoid arthritis (RA) who have insufficient or intolerable response to methotrexate. This product is a Janus kinase inhibitor, which is taken twice a day. On November 6, 2012, the food and Drug Administration (FDA) and Pfizer jointly announced that tofatinib citrate was approved for adult patients with moderate to severe active rheumatoid arthritis (RA) who had insufficient or intolerable response to methotrexate treatment. Xeljanz can be used as a single treatment or in combination with methotrexate or other nonbiological disease relief anti rheumatism drugs (DMARD). The drug should not be used in combination with biological DMARD or strong immunosuppressants such as azathioprine and cyclosporin. The approved dose of xeljanz was twice a day, 5mg per time. Seven clinical trials evaluated the safety and effectiveness of tofatinib citrate in adults with moderate to severe active rachemicalbook. In all trials, patients treated with xeljanz had significant improvement in clinical response and physical function compared to those who received placebo. In clinical trials, the most common adverse reactions were upper respiratory tract infection, headache, diarrhea, nasal congestion, throat pain and nasopharyngitis. The use of xeljanz is associated with increased risk of serious infection, including opportunistic infections, tuberculosis, cancer and lymphoma. The xeljanz product label contains framed warnings about these security risks. The treatment of xeljanz was also related to the increase of cholesterol and liver enzyme values and the decrease of blood cell count. To study the long-term effects of xeljanz on heart disease, cancer and severe infections, FDA requires a post market study that will assess two doses of xeljanz (tofatinib citrate) treatment and include it in a control group that received another approved treatment.
Our Tofacitinib API and Intermediates is as following,we have all the below API and intermedites offering all year.
Tofacitinib Intermediate Cas 3680-69-1,Tofacitinib API Cas 540737-29-9
Tofacitinib Intermediate Cas 3680-69-1,Tofacitinib API Cas 540737-29-9,Tofacitinib Intermediate Preparation Manufacturers and Suppliers in China
 Shandong Haohong Biotechnology Co., Ltd. , https://www.haohongpharma.com